The Drug Discovery Industry is Facing a Crisis
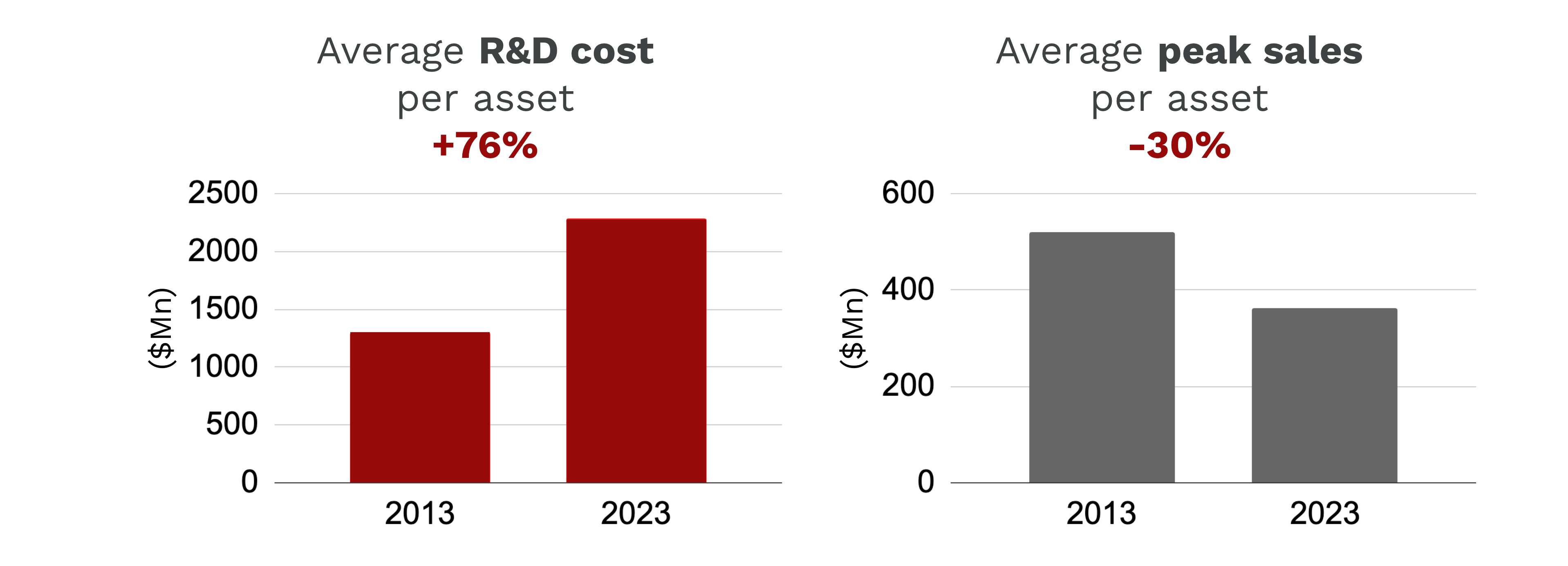
A report by Deloitte to measure the return on pharmaceutical innovation highlights a troubling trend from 2013 to 2023:
- Average R&D Costs per asset have increased by 76%.
- At the same time Average forecast peak sales per assets fell by 30%.
This trajectory is of course not sustainable. We can’t keep spending more on developing drugs while earning less. To reverse course, we must confront the root causes driving this imbalance.
Discovery is Forced to Move Beyond Small Molecules
One hypothesis of rising R&D costs is that all the low hanging fruit in this gold mine of small molecules has already been commercialized. Pharma has thus needed to expand beyond small molecules to biologics like Wezlana or cell therapies like CAR-T or stem cell therapies. In the 2000s, the majority of products in the pharma pipeline came from small molecules with 15% from cell therapies and biologics. That segment has now grown to 50%.
On top of all this, studies now increasingly use more complex disease models like tissues that co-culture many different cell types. The FDA has announced a plan to replace animal testing with more effective, human-relevant methods, including “ toxicity testing” on human-relevant tissues in April of 2025, followed by the NIH announcing an end to funding for animal-only studies in July of 2025, and just today the NIH announced nation's first dedicated organoid development center to reduce reliance on animal modeling. All with continued support from the current administration.
In summary, the complexity of discovery is increasing in both therapy modalities as well as in disease modeling. This means growing and experimenting on living cells is becoming increasingly important which dramatically changes the workflows of scientists.
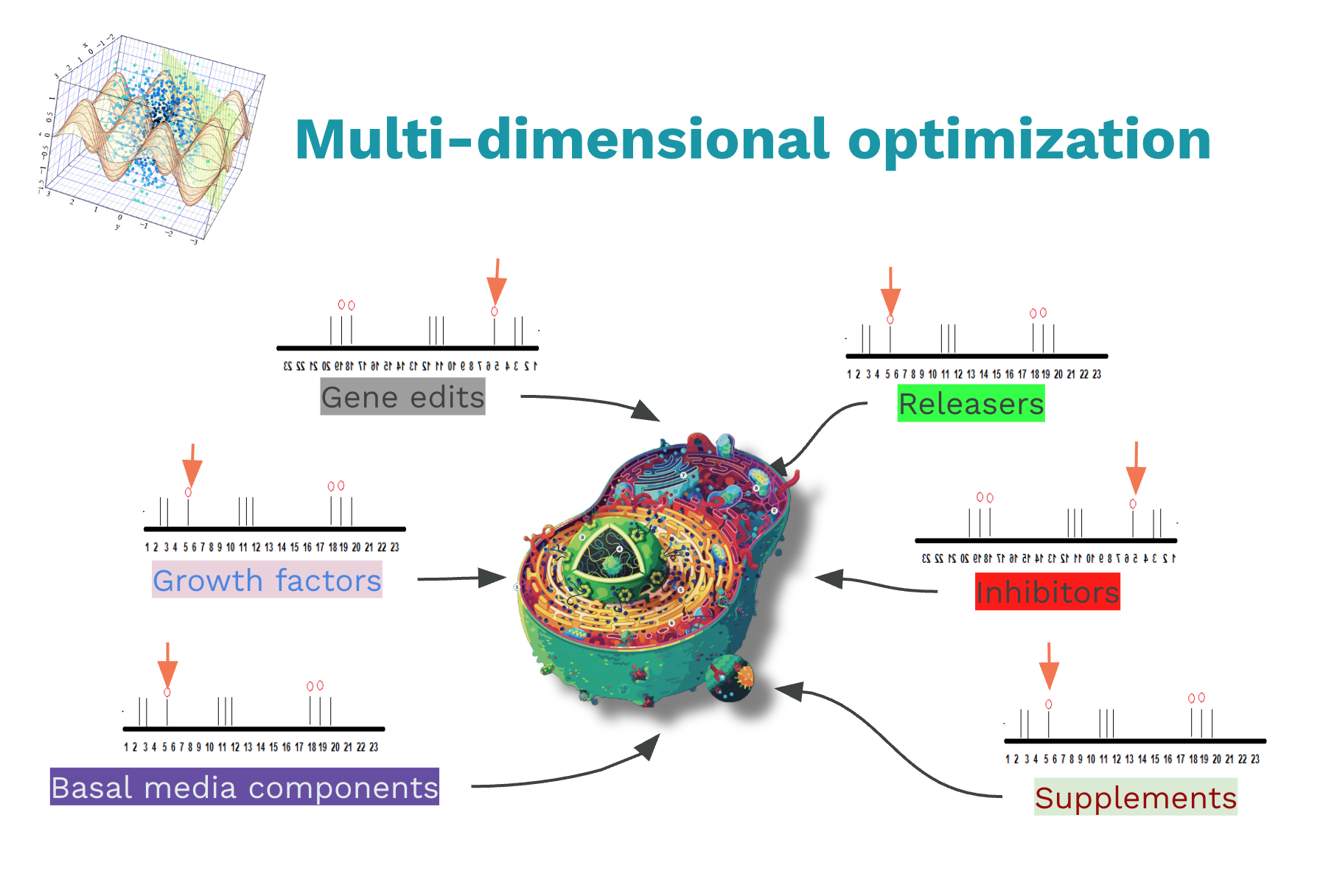
Cell-based Experiments Have a Huge Search Space
Workflows that involve cell based experiments have a huge search space. Scientists want to try many different parameters along many different axes. In the 2000s, traditional lab automation has addressed workflows for high throughput screening in which the goal is to find that one small molecule in a big library of a million molecules. These are what I call one dimensional searches. Today we have to engineer living cells. Try 1000s of CRISPR gene edits, vary growth factors at different dilutions and apply them at various time points, and screen many different media compositions.
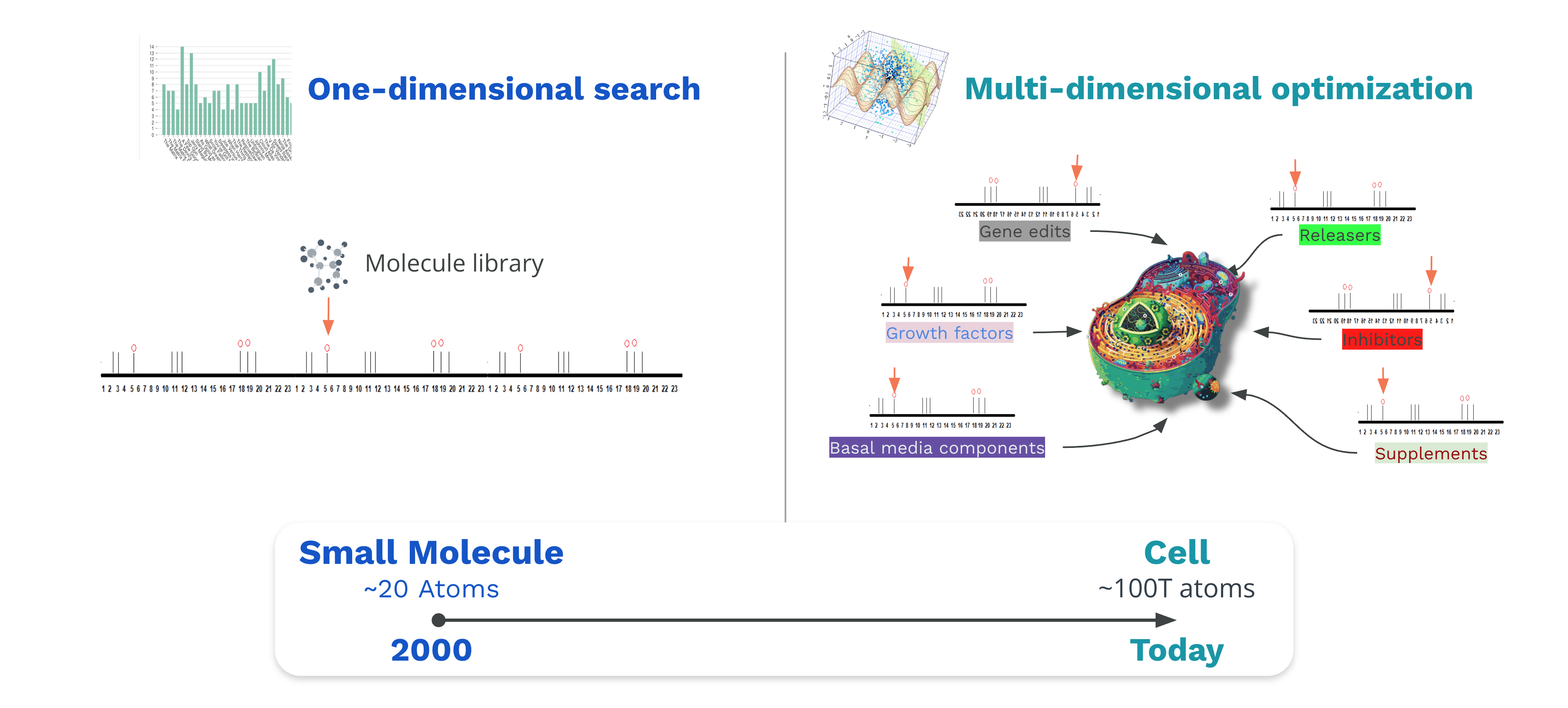
I call this multi-dimensional search. This has resulted in a combinatorial explosion of possibilities to try within this massive search space. To traverse this search space we need new tools.
Growth of AI-enabled Biotech is Powered by 3 Technologies
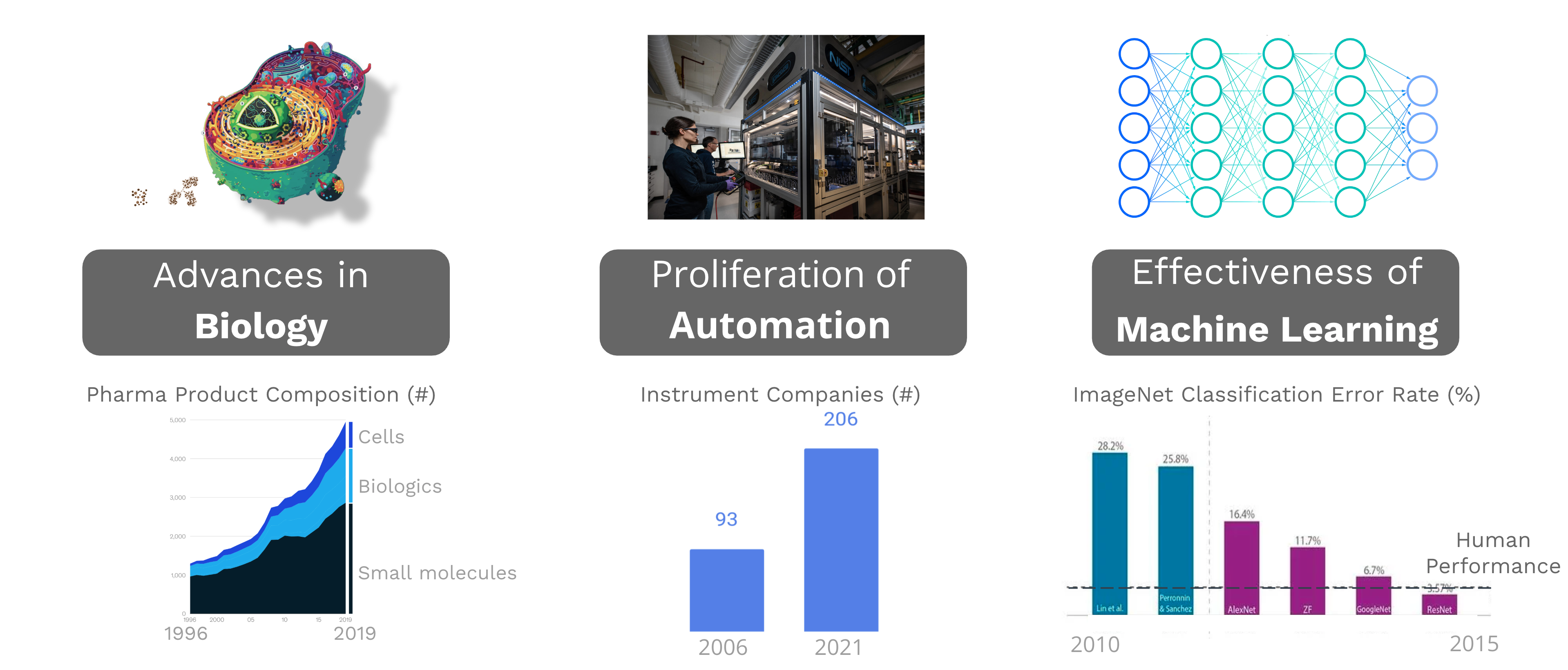
Enter what many refer to as AI-enabled biotech. This movement is powered by three key trends:
- Advances in biology. With industry moving from small molecules to biologics to cells. I believe this trend is what drives the next two tends.
- Proliferation of automation. There is now a need for automation to enable throughput sufficient to search a larger solution space. Which is why we’ve seen a proliferation of automation in terms of the number of instrument vendors. The number has more than doubled in the past 15 years. On top of that each vendor is offering more types of modalities that can generate different types of data.
- Effectiveness of Machine Learning. Some models now surpass human performance. Adoption of ML now makes it more important than ever to capture experimental data and ensure they are high quality for subsequent input into these pipelines.
And has been growing steadily. Some of these AI-enabled biotech companies emphasize lab automation to run biology at scale and reduce costs. Others emphasize data management and machine learning. Still others like Recursion Therapeutics and Insilico Medicine leverage both approaches reporting massive productivity gains.
AI-enabled Biotech Reverses the Trend of Increasing Costs
Recursion is a great case study showing us the AI-enabled biotech approach can reverse this decade long trend of steadily increasing costs per asset. By leveraging automation with ML and increasingly using foundational models they can
- Test MORE candidates: broaden the number of potential therapeutic starting points.
- Eliminate candidates EARLIER: rapidly identify failures earlier in the research cycle when they are relatively inexpensive.
By taming the shape of their drug discovery pipeline, Recursion reports they can decrease cost to IND filing per asset by about 3X. According to Chris Gibson, CEO of Recursion, they were able to go from novel target biology to preclinical drug candidate in under 18 months, nearly twice the speed of the industry average.
That’s just one company. There’s an ecosystem of tools being built to help more labs become AI-enabled biotechs.
Next
Building AI-enabled biotechs and transforming the industry is a long-term journey, not an overnight shift. In my next blog post, I’ll outline a technology roadmap and present a framework for charting the progression toward increasingly intelligent labs—leading to the long-term vision of autonomous self-driving labs and, ultimately, scientific superintelligence.