Cell Culture
At Monomer Bio, our work centers on cell culture–based protocols, beginning with some of the most complex and demanding: iPSC-derived in vitro disease modeling. We deliberately chose this niche—arguably one of the hardest workflows—on the principle that software provides the biggest lever here to manage the finicky and complex nature of growing stem cells. And if we can master stem cells, we can extend the approach to virtually any other cell or tissue type.
For each level of autonomy I’ll break down how cell culture is performed. Then I’ll describe how our software platform helps in those various levels upgrading from manual to semi-automated to fully-automated, and finally acting as a reinforcement learning environment for AI companies to train, fine-tune, and validate their foundation models with real biology experiments. I’ll end each section describing what new bottleneck emerges.
Level 0: Manual
At Level 0, everything is done by hand. The scientist is the “automation system.” Pipettes, flasks, multi-well plates, and microscopes are used entirely manually. Every step from seeding cells, exchanging media, staining, focusing and positioning the sample for microscopy to documenting the process is performed directly by the researcher. Documentation is typically done with pen-and-paper or ad-hoc spreadsheets as the only record-keeping tools.
Steps that are repeated daily are:
- Media changes: measured and pipetted by hand.
- Passaging: manually detaching and replating cells. This is often done with variable timing and technique depending on the scientist.
- Making observations: eyeballing cells under a microscope and writing down notes like “70% confluent,” “healthy”, “contaminated”, “dry”, etc.
- Documenting process details: images may be taken and are typically organized in ad hoc folder structures and filename schemas with little linkage to metadata or process history.

Level 0 represents the traditional state of biology. Workflows are highly flexible. Scientists can improvise and adapt on the fly but the trade-off is low throughput, high variability, and poor reproducibility.
Monomer streamlines the documentation process by pairing our software with a microscope like the Agilent Cytation or Cephla Squid. This approach saves documentation time, ensures consistent data recording, and makes information readily available in digital format. But all the pipetting and moving of plates is still performed manually by hand.
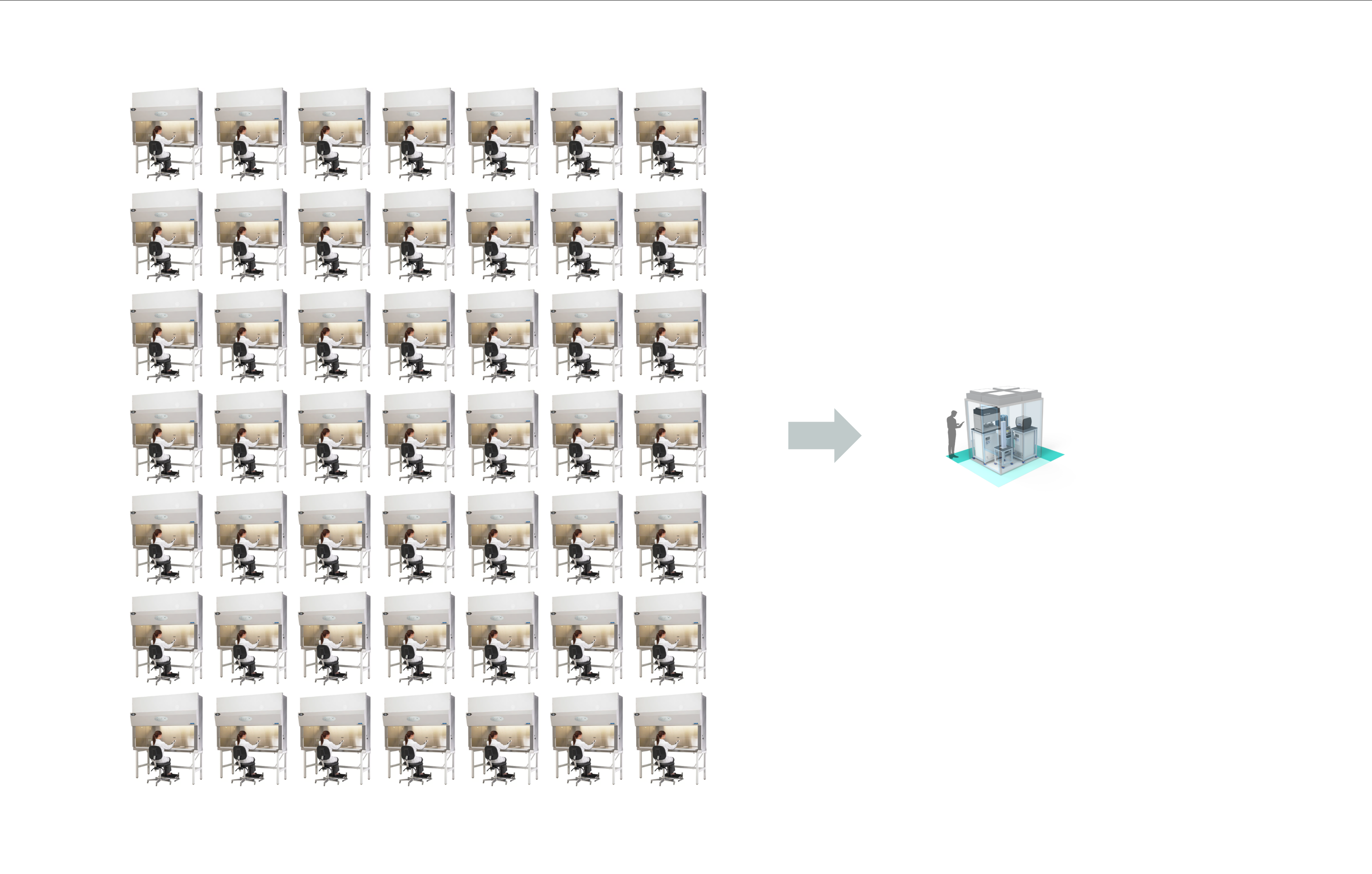
In practice, manual workflows limit one researcher to managing just a few flasks or plates at a time, one tissue type at a time, and making complex or large-scale experimentation infeasible. At one big pharma for example, I’ve counted 300 biosafety cabinets for manual cell culture.
In this mode, discovery relies almost entirely on individual craftsmanship. The scientist’s intuition and dexterity are central, but results are difficult to scale, share, or reproduce. This is the natural starting point for nearly every lab — and also where the journey toward autonomy begins.
The first step toward autonomy is offloading some of that physical labor onto machines.
Level 1: Lab Automation
Level 1 introduces assistance in the form of what we traditionally refer to as lab automation. The goal is not to change what scientists do, but exchanging repetitive manual steps for reliable automation steps. This is the point where robotics enters the lab, but the intelligence remains human.
Instruments are introduced to automate unit operations like pipetting, taking microscopy images across all the wells in a plate, and storing cells in a 37 Celsius incubator. A popular place to start is a walk up automated liquid handler for robotic media changes.
Monomer’s scheduler is designed for orchestrating cell culture across all those instruments and excels in four key areas:
- Managing multi-day workflows with complex branching and decision points.
- Orchestrating consumable management to bring out just the right amount of media from the fridge at the perfect time for pre-warming.
- Enabling individual well-level control for changing media or passaging specific wells.
- Text based programming of protocols making it version control-friendly and editable with AI tools like Claude or Cursor to make developing complex protocols effortless.
At this stage, the focus is entirely on executing steps reliably and quickly. Staining steps or image acquisitions steps can take on the order of hours so there are often steps that have to be started in the middle of the night. You won’t be able to do that with humans regularly for long. The workcell makes it easy to run steps back to back and have consistent timing. Additionally, contamination doesn’t happen anymore! Most contamination comes from the human user. So the less exposure to breathing or touching, the better to keep sterility.
However, as you increase throughput with Level 1 automation a new bottle neck emerges: scientists may generate more results than they can meaningfully interpret. If you’re not careful you can easily end up with disorganized folders of images and scattered experimental metadata.
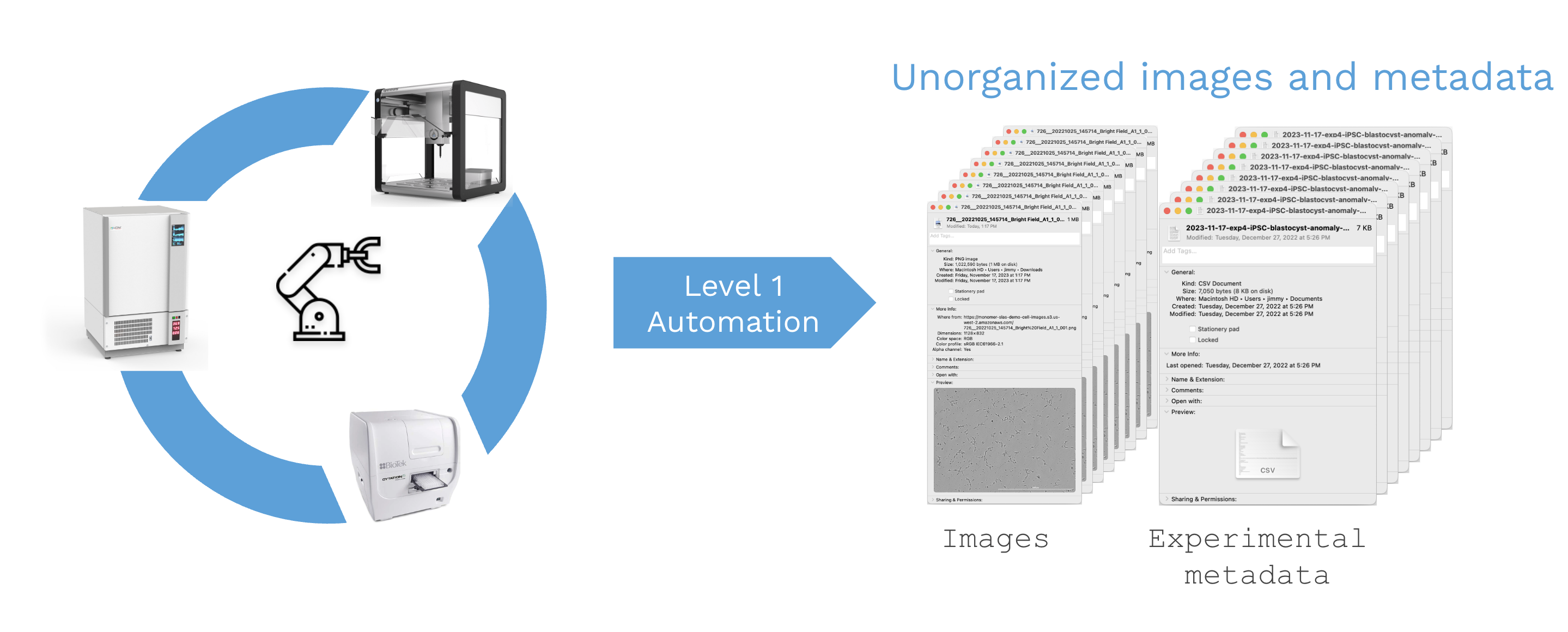
For simple workflows, manual data management might be sufficient, but this becomes woefully inadequate for complex cell-based experimentation or AI-enabled biotech approaches. In other words, Level 1 automates physical work but does not help with the large amounts of data it then produces.
Level 2: Data-centric Automation
Level 2 closes the gap between doing and observing. The challenge shifts from executing physical tasks to managing the torrent of data those tasks produce. For cell culture, this generally means keeping tabs on cell numbers from a cell counter, images from a microscope, and other numeric data from a flow cytometer or platereader.
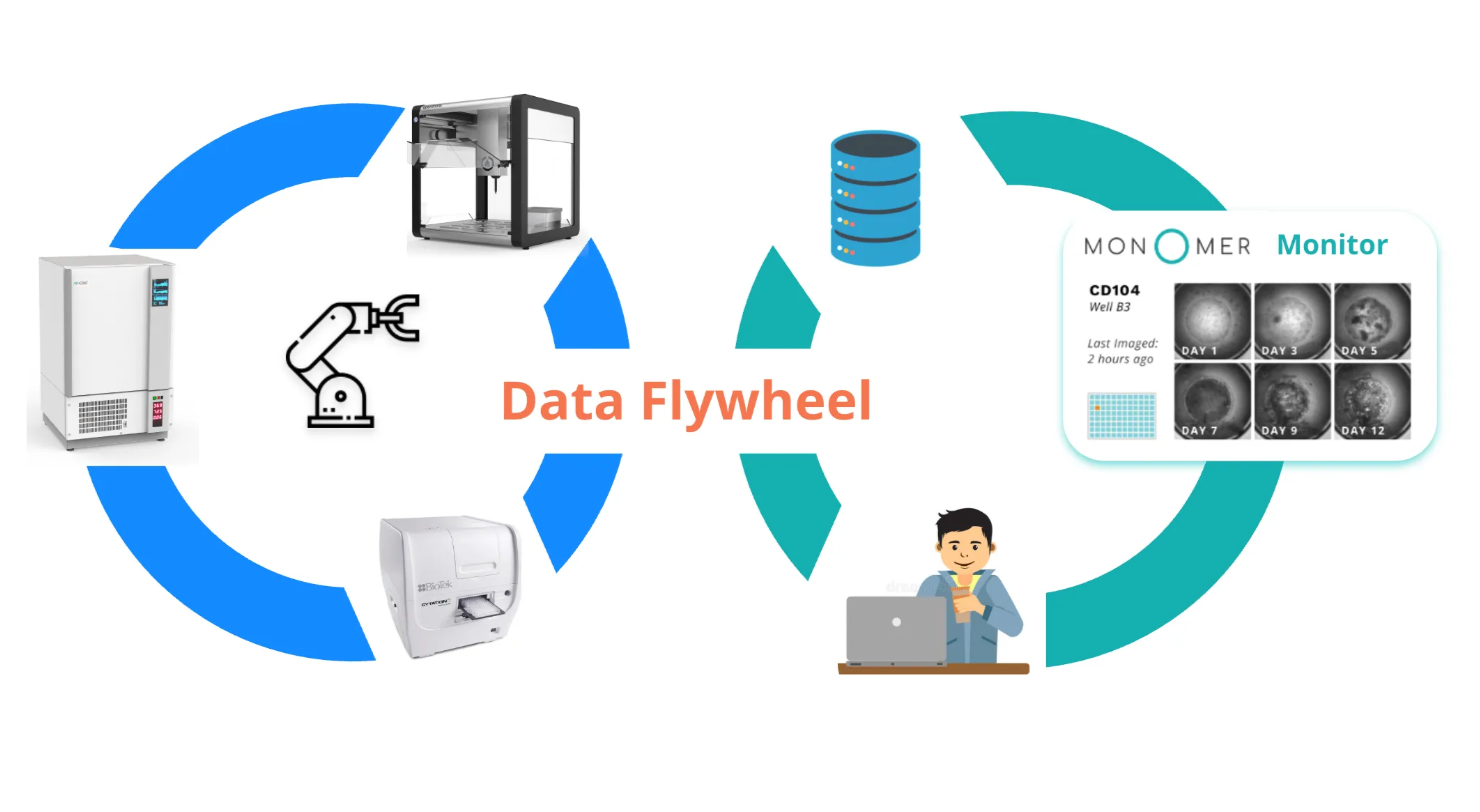
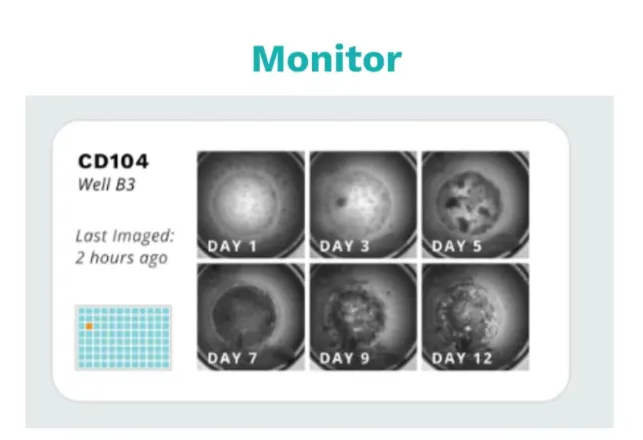
Monomer Bio’s platform automatically aggregates that data while also keeping track of experimental metadata like seeding times and passaging decisions as scientists grow cells. It organizes these in a cell-centric database that powers our Culture Monitor UI which scientists can use to easily review cell health across all their cell lines. The easier it is to access data across all your cell lines, the more cells you can grow at a time.
With microscopy images readily accessible, you can make near real-time decisions and significantly increase the number of finicky cell lines you can manage simultaneously. To give a concrete example, Rosebud Biosciences can culture four tissue types—brain, cardiac, liver, and lung—at a scale of 1,920 organoids simultaneously, all maintained by a single research associate.

This stage is where biology becomes quantifiable. Every cell culture is represented digitally tracking every image, media change, passage, and lineage. Trends once buried in notebooks become visible in dashboards. With this visibility, labs start to behave more like manufacturing systems that aremeasurable, monitorable, and improvable. However, as data volume grows, reviewing all that data becomes the bottle neck. This is where AI/ML becomes essential.
Level 3: ML-guided
Level 3 introduces perception, the ability for machines to see and interpret biological phenomena. At this stage, machine learning models analyze raw data such as images and generate growth observations, transforming them into actionable insights. Rather than manually inspecting every well or data point, scientists can rely on algorithms to flag cultures that need attention.
Computer vision now quantifies cell morphology and confluence objectively, while statistical control charts highlight trends or deviations that fall outside expected ranges. Instead of spending hours scanning for anomalies, scientists can focus on the exceptions that truly matter.
Monomer’s ML assistant exemplifies this level of autonomy. It automatically detects wells showing potential contamination, growth trajectories outside expected thresholds, or cultures approaching confluence that require passaging. By doing so, it eliminates the need to review thousands of images individually.

At this level, humans remain fully in the loop. The scientist still decides what to try next — guided by intuition, experience, and scientific reasoning — but now supported by a digital assistant that perceives subtle patterns across thousands of wells that no human could track unaided.
In essence, Level 3 augments human judgment with machine perception. It’s a partnership: the machine surfaces insights, and the scientist interprets and acts on them. Level 4 builds on this foundation — where algorithms begin to not only suggest, but also decide what experiments to run next.
Next
So far we've covered automated cell culture from Level 0 to Level 3. In the next post I’ll continue our journey fleshing out level 4 and 5 to describe autonomous cell culture.